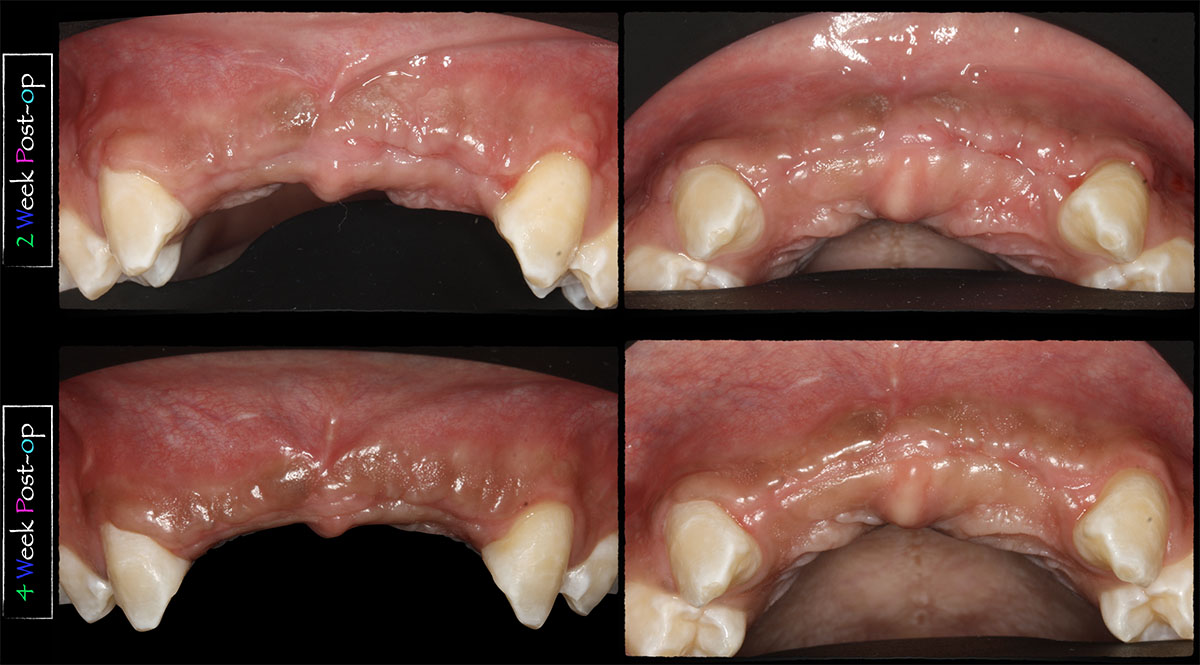
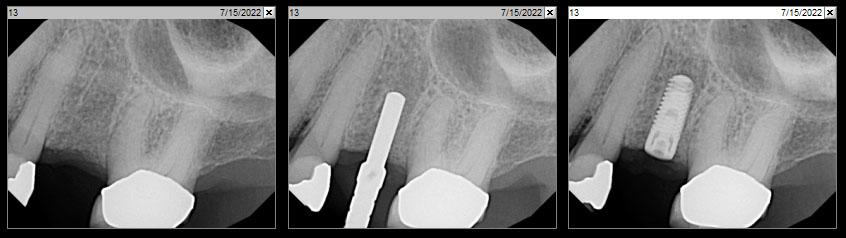
What is Geistlich vallomix™?
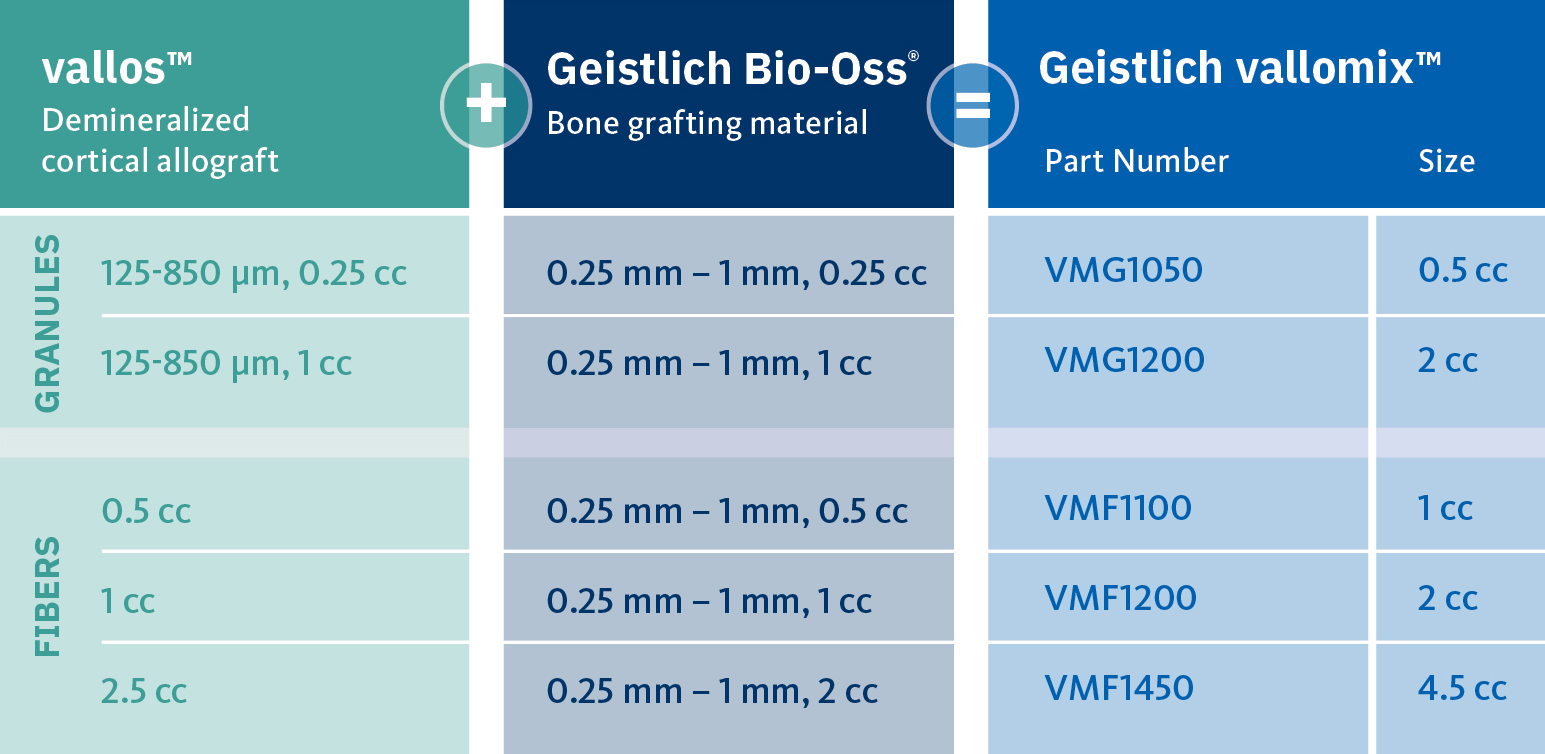
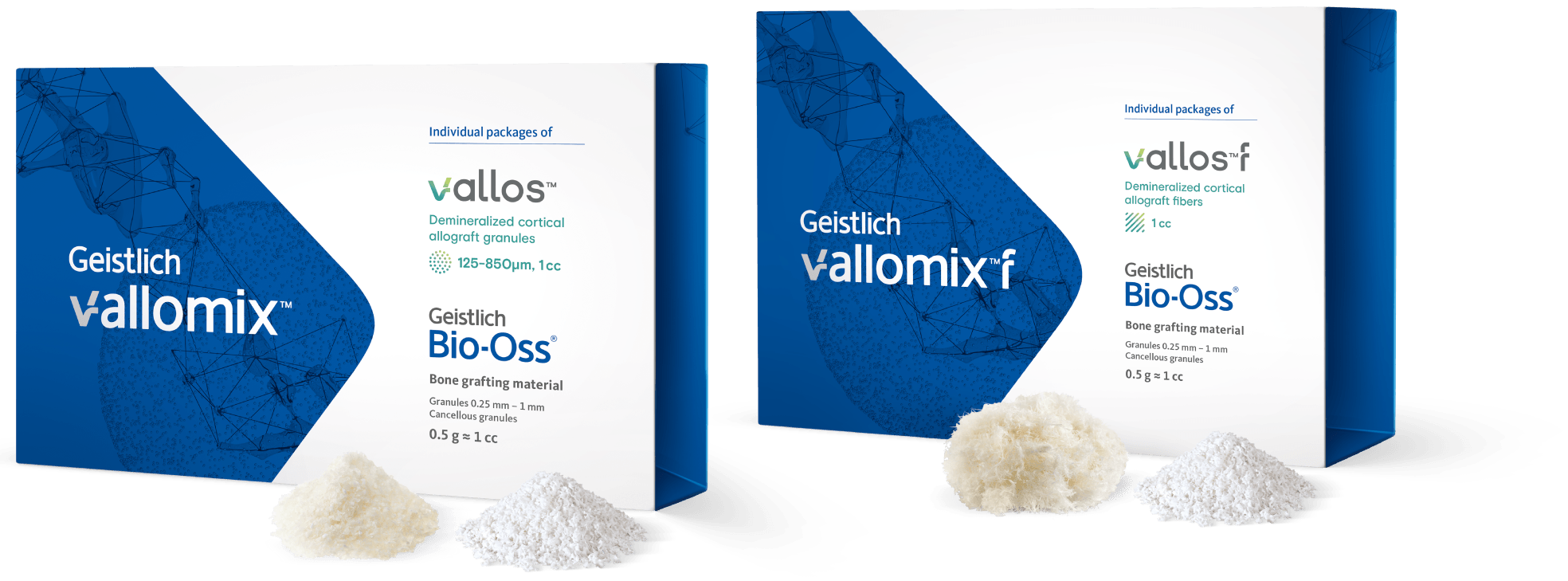
Geistlich vallomix™ is the first conveniently co-packed xenogeneic / allogeneic validated bone substitute1. Geistlich vallomix™ contains individually packaged Geistlich Bio-Oss® granules and vallos® granules or fibers. Together, these products offer the opportunity to achieve optimal consistency and performance in demanding clinical situations.
- Compiled DCI Osteoinductivity Validation Summary_2020. (data on file)
What are the main benefits of Geistlich vallomix™?
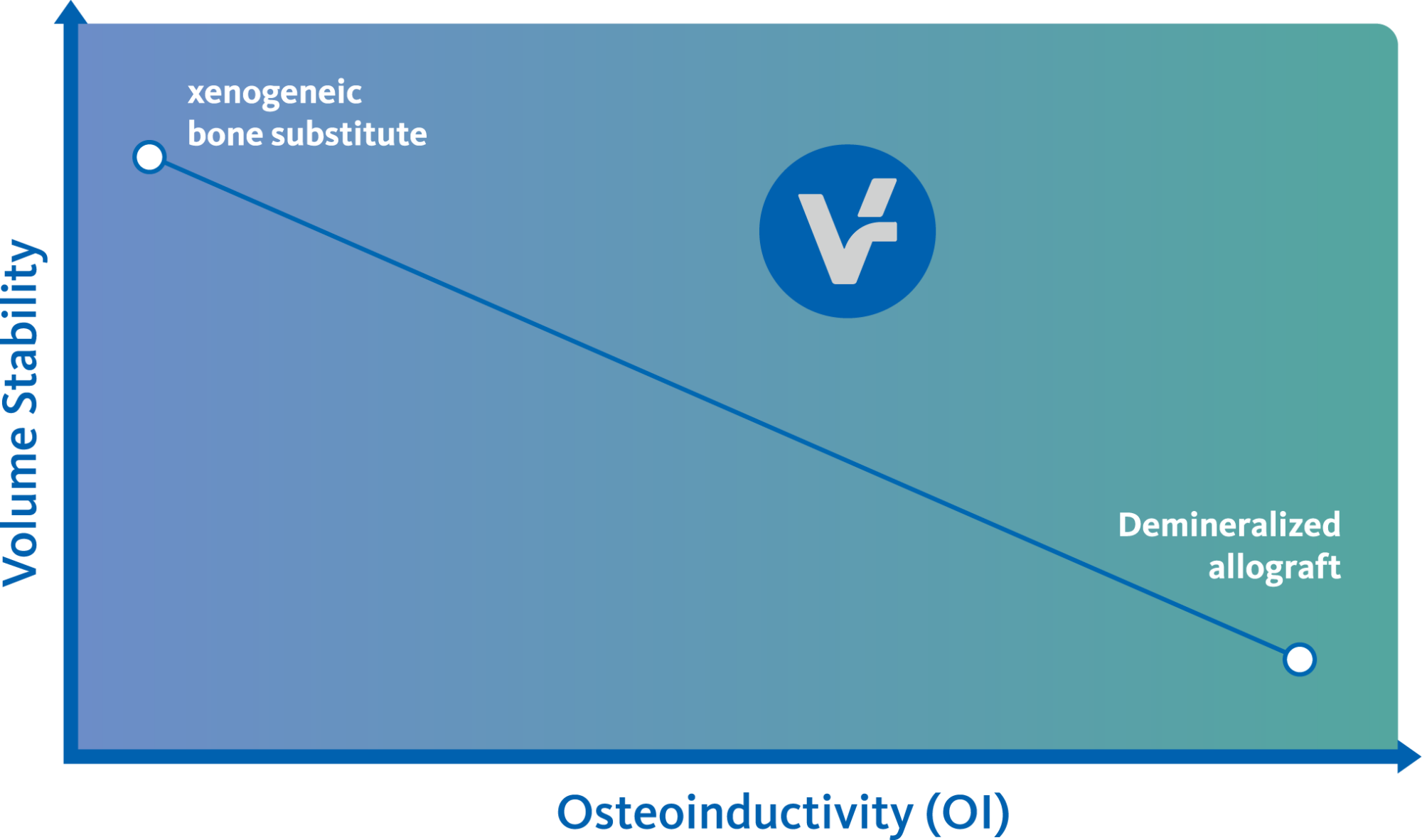
The combination of a validated allograft with osteoinductive potential1 and a volume preserving xenogeneic bone substitute can provide significant advantages in regenerative procedures, enabling the possibility of reduced time between tissue regeneration and implant placement. Geistlich vallomix™ simplifies combining a high quality demineralized cortical allograft, vallos® with Geistlich Bio-Oss®, the leading xenogeneic bone substitute for regenerative dentistry worldwide2,3, with more than 35 years of scientific validation.
- Compiled DCI Osteoinductivity Validation Summary_2020. (data on file)
- iData Research Inc., US Dental Bone Graft Substitutes and other Biomaterials Market, 2015.
- iData Inc., European Dental Bone Graft Substitutes and other Biomaterials Market, 2015.
What is the difference between vallos® and vallos®f?
vallos® is an allogeneic bone in the form of granules, whereas vallos®f is an allogeneic bone in the form of fibers (shavings from cortical bone). vallos®f shows putty-like handling properties and can be easily molded by the clinician into a desirable shape for application to the defect site. vallos®f has also demonstrated 4-times higher osteoinductive potential compared to vallos®.1 Both vallos® and vallos®f can be mixed with Geistlich Bio-Oss® in a dry or hydrated state.
- Compiled DCI Osteoinductivity Validation Summary_2020. (data on file)
How does vallos® or vallos®f differ from other allografts?
vallos® and vallos®f are demineralized cortical freeze-dried allografts that offer osteoinductive potential and new vital bone formation after 18-20 weeks.1 Our allograft supplier meets the highest quality standards, is a federally certified organ procurement organization, and remains in control of all processes, from donor screening to all steps of allograft processing, to provide a state-of-the-art product. To counterbalance heterogeneity between different donors, every donor lot for vallos® is tested to ensure osteoinductive potential. Moreover, adding Geistlich Bio-Oss® offers the potential for more consistent and predictable clinical outcomes.
- Robert A Wood & Brian L Mealey: J Periodontol. 2012 Mar;83(3):329-36. (Clinical Study)
Can vallos® and vallos®f be purchased separately from Geistlich vallomix™?
These allografts are sold separately under our vallos® product line. Please reference the product line here.
Why combine a demineralized allograft with Geistlich Bio-Oss®?
Demineralized allografts offer the potential of osteoinductivity1-3 as compared to mineralized allografts. While offering new bone formation capacity, demineralized bone matrices are subject to volume resorption during the healing process.4 Geistlich Bio-Oss® has shown to consistently maintain grafted bone volume over time.5-10 Thus, the demineralized vallos® and Geistlich Bio-Oss® complement each other to obtain a bone graft that has osteoinductive potential while maintaining its volume.
- Glowacki, J., S. Zhou, and S. Mizuno, J Craniofac Surg, 2009. 20 Suppl 1: p. 634-8
- Glowacki, J Oral Maxillofac Surg . 2015 Dec;73(12 Suppl):S126-31. doi: 10.1016/j.joms.2015.04.009.
- Urist, Marshall R. (1965). Science 12:150 (698): 893–899.
- Serrano CA et al.: Implant Dent. 2018 Aug; 27(4):467-473. (Clinical Study).
- Piattelli M et al.: Int J Oral Maxillofac Implants 1999; 14 (6), 835-40. (Clinical Study).
- Sartori S et al.: Clin Oral Implants Res 2003; 14 (3), 369-72. (Clinical Study).
- Maiorana C et al.: Int J Periodontics Restorative Dent 2005; 25 (1), 19-25. (Clinical Study).
- Orsini G et al.: Oral Dis 2007; 13 (6), 586-93. (Clinical Study).
- Lindgren C et al.: Int J Oral Maxillofac Implants 2009; 24 (6), 1093-100. (Clinical Study).
- Mordenfeld A et al.: Clin Oral Implants Res 2010; 21 (9), 961-70. (Clinical Study).
What makes vallos® and vallos®f validated allografts?
Numerous studies show that allografts demonstrate a high degree of heterogeneity that is associated with inter-donor variability.1-2 Inclusive of the state-of-the-art quality control and donor safety assessments, vallos® and vallos®f are both tested for every donor lot on its osteoinductive potential. This offers the opportunity to obtain more predictable clinical outcomes3. This ensures that each lot has been processed, tested, and validated for osteoinductive potential, reducing lot-to-lot variability.
- Urist, Marshall R. (1965). Science 12:150 (698): 893–899.
- Schwartz et al., J Periodontol. 1996 Sep;67(9):918-26. doi: 10.1902/jop.1996.67.9.918
- Serrano CA et al.: Implant Dent. 2018 Aug; 27(4):467-473. (Clinical Study).
How is osteoinductive potential tested?
The osteoinductive potential is assessed in vitro with the alkaline phosphatase activity (ALP) testing method on every donor lot for vallos® and vallos®f. ALP is a commonly used indicator for bone formation processes in synthesizing hydroxyapatite. The acceptance criteria for vallos® and vallos®f are based on a measurement according to an osteoinductivity index, above which a donor lot can be considered osteoinductive positive based on the in vitro and in vivo (heterotopic bone formation) correlation studies1-2.
- Compiled DCI Osteoinductivity Validation Summary_2020. (data on file)
- Han et al., 2003, J Orthop Res 21_648-654
What clinical evidence is available for combining allogeneic and xenogeneic bone?
The combination of allogeneic and xenogeneic bone has been well documented and widely used in clinical practice for many years1-2. Studies specific to Geistlich vallomix™ are in preparation.
- Serrano CA et al.: Implant Dent. 2018 Aug; 27(4):467-473. (Clinical Study).
- Landi L. et al., Int J Periodontics Restorative Dent. 2000 Dec;20(6):574-83. PMID: 11203594.
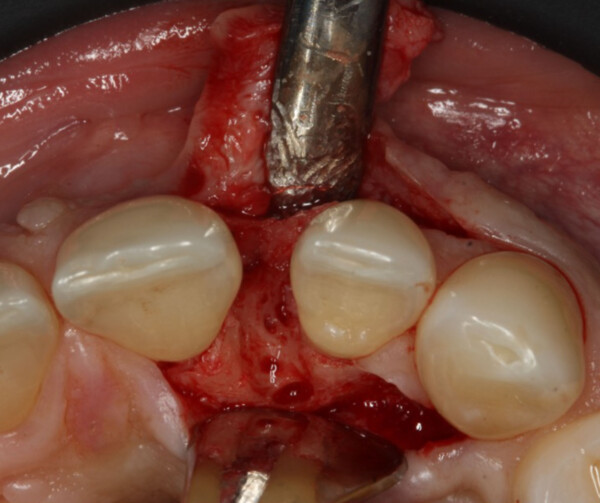
Should Geistlich vallomix™ be mixed or layered?
Geistlich vallomix™ can be mixed, layered, or adjusted based on the clinical needs of the patient, providing flexibility for a range of therapeutic areas.
Do Geistlich vallomix™ and Geistlich vallomix™f require preparation prior to use?
It is recommended to hydrate and mix Geistlich Bio-Oss® along with the vallos® or vallos®f component. According to the package insert, vallos® should be hydrated for a minimum of 30 minutes. When preparing vallos®f gently mix for 30-60 seconds until completely hydrated. It is recommended that all freeze-dried allografts be hydrated in Ringer’s solution, normal saline, or other normal physiologic solution containing antibiotics of the clinicians’ preference. The decision to hydrate freeze-dried bone should be based on the clinician’s preference. Please see the Package Insert for more information.
Can Geistlich vallomix™ replace the combination of demineralized and mineralized allografts?
Clinician preference dictates which choice in biomaterials should be used. Geistlich vallomix™ embodies a combination of demineralized and mineralized bone material and can be considered a suitable replacement of demineralized and mineralized allograft material. Geistlich Bio-Oss®provides the mineralized component of Geistlich vallomix™, which has been attributed to beneficial volume stability over mineralized allografts1.
- Lee et al., Int J Oral Maxillofac Implants. 2009 ; 24 :609-615